Seasonal Influenza 2019/2020
Information on the vaccines approved in Germany, the approved vaccine doses and the vaccine base composition for the 2019/2020 season
Vaccines
The basic composition of the influenza vaccines has to be adapted to the current epidemiological situation every year because the properties of the circulating influenza viruses change. The exact composition is determined each year by the World Health Organization (WHO). This adjustment is checked for each vaccine in a procedure to change the approval. After approval of the strain adaptation, the Paul-Ehrlich-Institut tests and releases the manufactured influenza vaccines in batches, which can then be put on the market and used.
Released Vaccine Doses
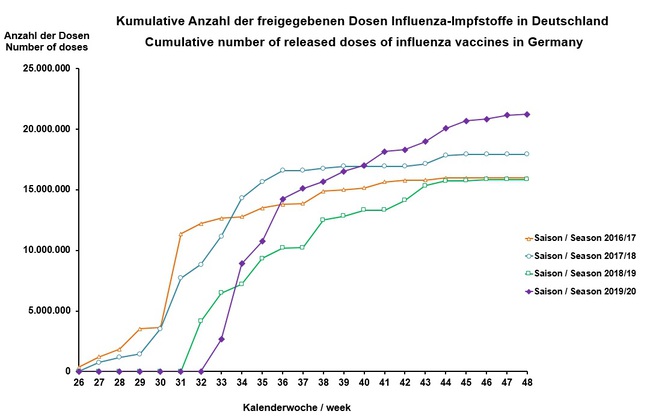
 Stand/ as of: 29.11.2019
Source: PEI / Source: PEI
Stand/ as of: 29.11.2019
Source: PEI / Source: PEI
| Date | Calendar Week | Number of Vaccine Doses |
|---|
| 16.08.2019 | 33 | around 2,7 Million |
| 23.08.2019 | 34 | around 8,9 Million |
| 30.08.2019 | 35 | around 10,7 Million |
| 06.09.2019 | 36 | around 14,2 Million |
| 13.09.2019 | 37 | around 15,1 Million |
| 20.09.2019 | 38 | around 15,7 Million |
| 27.09.2019 | 39 | around 16,5 Million |
| 04.10.2019 | 40 | around 17 Million |
| 11.10.2019 | 41 | around 18,2 Million |
| 18.10.2019 | 42 | around 18,3 Million |
| 25.10.2019 | 43 | around 19 Million |
| 01.11.2019 | 44 | around 20 Million |
| 08.11.2019 | 45 | around 20,7 Million |
| 15.11.2019 | 46 | around 20,8 Million |
| 22.11.2019 | 47 | around 21,1 Million |
| 29.11.2019 | 48 | around 21,2 Million |
Composition of Influenza virus Vaccines 2019/2020
The influenza vaccine for the 2019/2020 season is composed of the antigens of globally circulating variants of the following viruses in accordance with the recommendations of the WHO and the Committee for Medicinal Products for Human Use (CHMP) at the European Medicines Agency (EMA):
- A/Brisbane/02/2018 (H1N1) pdm09- like virus
- A/Kansas/14/2017 (H3N2)- like virus
- B/Colorado/06/2017- like virus (B/Victoria/2/87-Linie)
For quadrivalent vaccines, the antigens of the above-mentioned viruses and a variant of B / Phuket / 3073/2013 - like virus (B / Yamagata / 16/88 line) - are recommended.
A and B denote the virus types, the place name refers to the place of virus isolation; the first digit indicates the number of the isolated strain, the second refers to the year of isolation. H and N abbreviate the two most important proteins in the virus envelope hemagglutinin and neuraminidase, the number behind them denotes the current hemagglutinin and neuraminidase subtype.
This composition differs from that of the 2018/2019 season.
Strains suitable for the Production of the Flu Vaccines in the 2019/2020 Season
| WHO Recommendations for the northern hemisphere | Suitable strains according to the EMA recommendations for influenza vaccines | Suitable strains according to the EMA recommendations for live attenuated influenza vaccines |
|---|
| A/Brisbane/02/2018 (H1N1) pdm09- like virus | A/Brisbane/02/2018, IVR-190 (egg-based)
A/Idaho/07/2018, Wildtyp (cell-based) | A/Switzerland/3330/2017, MEDI307134 |
| A/Kansas/14/2017 (H3N2)- like virus | A/Kansas/14/2017, NYMC X-327 (egg-based)
A/Indiana/08/2018, Wildtyp (cell-based) | A/Kansas/14/2017, MEDI308763 |
| B/Colorado/06/2017- like virus (B/Victoria/2/87-lineage) | B/Maryland/15/2016, Wildtyp (egg-based)
B/Maryland/15/2016, NYMC BX-69A (egg-based)
B/Iowa/06/2017, Wildtyp (cell-based) | B/Colorado/06/2017, MEDI293454 |
| B/Phuket/3073/2013 - like virus (B/Yamagata/16/88-lineage) | B/Phuket/3073/2013, Wildtyp (egg-based)
B/Brisbane/9/2014, Wildtyp (egg-based)
B/Utah/9/2014, Wildtyp (egg-based)
B/Phuket/3073/2013, BVR-1B (egg-based)
B/Singapore/INFTT-16-0610/2016, wild type (cell-based) | B/Phuket/3073/2013, MEDI254977 |
top



