Typhoid Vaccine Typhim Vi with English/French/Spanish Labelling Available in Germany
Typhim Vi is a travel vaccine against typhoid fever. The marketing authorisation holder, Sanofi Pasteur Europe, has announced that the expected demand for Typhim Vi in Germany cannot be met with the current stock levels and expected regular deliveries. Sanofi therefore intends to make Typhim Vi available on the German market with foreign labels from 3 July until 31 August 2023.
Details
- The foreign-labelled product also has the trade name Typhim Vi (see photos below).
- The imported batch is Typhim Vi batch U2A491V, to be used until 31 October 2023.
- The PZN is 18771519.
- The foreign-labelled product is a prefilled syringe with a fixed needle.
- The labelling on the syringe label and in the package leaflet is trilingual (FR, EN, ES), the labelling on the package is bilingual (FR, EN).
- The product is in its original form and does not contain a German package leaflet.
- The German package leaflet and summary of product characteristics are available for download on this page.
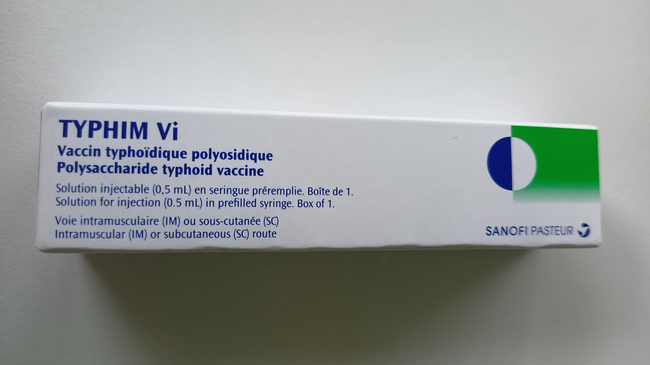 Front of the package
Source: Sanofi-Aventis Deutschland GmbH
Front of the package
Source: Sanofi-Aventis Deutschland GmbH
 French label on the side of the package
Source: Sanofi-Aventis Deutschland GmbH
French label on the side of the package
Source: Sanofi-Aventis Deutschland GmbH
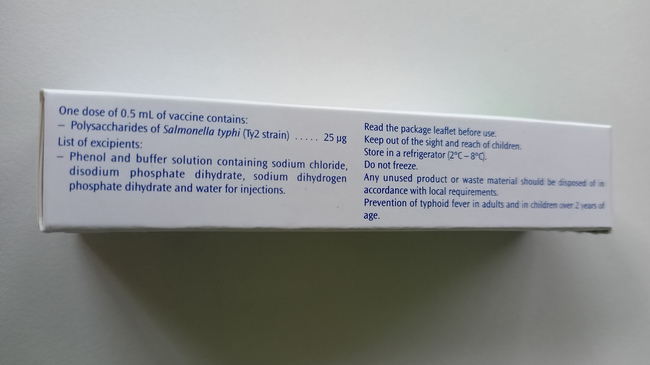 Label on the side of the package in English
Source: Sanofi-Aventis Deutschland GmbH
Label on the side of the package in English
Source: Sanofi-Aventis Deutschland GmbH
 Back of the package
Source: Sanofi-Aventis Deutschland GmbH
Back of the package
Source: Sanofi-Aventis Deutschland GmbH
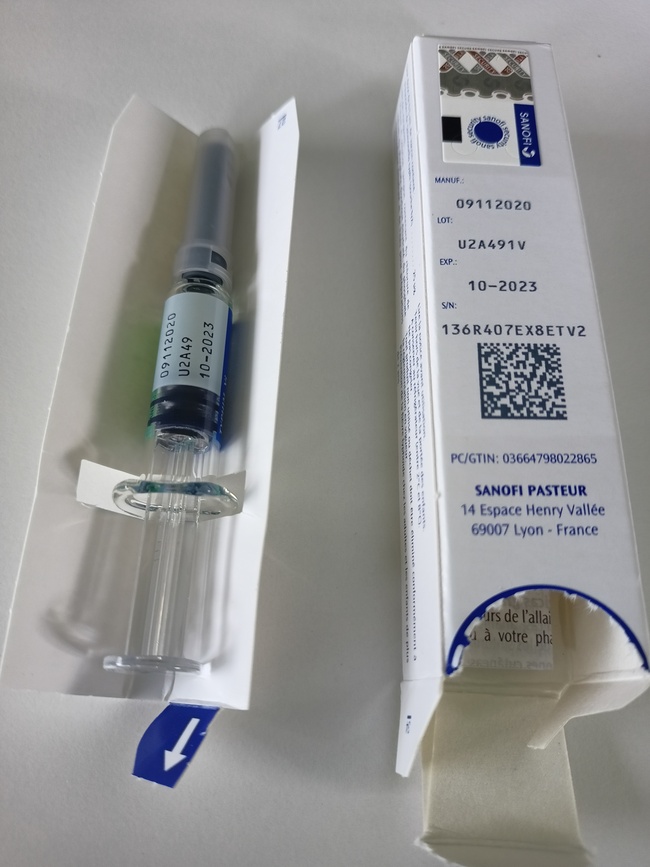 Contents of the package
Source: Sanofi-Aventis Deutschland GmbH
Contents of the package
Source: Sanofi-Aventis Deutschland GmbH
How should the vaccine be used?
The instructions listed in the package leaflet must be followed.
Background
The Paul-Ehrlich-Institut, the Federal Institute for Vaccines and Biomedicines, is the higher federal authority in Germany responsible for assessing the quality, safety and efficacy of vaccines in the context of marketing authorisation and national batch release. It also advocates for the availability of these medicines.
In the event of a critical supply shortage, section 4 (1) of the Medical Needs Supply Assurance Ordinance (MedBVSV) enables the Paul-Ehrlich-Institut to, in individual cases, authorise the marketing of medicinal products in Germany that do not comply with the labelling and package leaflet requirements of sections 10 and 11 of the German Medicines Act (e.g. foreign-language labelling). Section 4 (5) of the MedBVSV allows the Paul-Ehrlich-Institut, after a risk-benefit assessment, to grant exemptions from the marketing authorisation regulations in individual cases if this is necessary to ensure the supply of the population with medicinal products. The latter allows a product to be marketed with a presentation that differs from the German marketing authorisation.
The website of the Paul-Ehrlich-Institut provides a daily overview of current supply shortages for human vaccines in Germany.
Our email newsletter "Safety and Availability – Information on medicines safety, instruction notes on human vaccine supply shortages" provides regular information about current availability and supply shortages.







