Researchers of the Paul-Ehrlich-Institut confirm: no health risk from foreign viral contaminants in Rotarix vaccine
As published in spring last year, the live viral vaccine Rotarix, manufactured by GlaxoSmithKline Biologicals, was found to be contaminated with components of a pig virus called Porcine circovirus type 1, (PCV1).When this knowledge became public, a group at the Paul-Ehrlich-Institut performed its own experiments to identify whether this contamination would affectthe safety of the vaccine. Dr Sally Ann Baylis and Dr Johannes Blümel, in collaboration with colleagues at the Robert Koch-Institut, demonstrated that, although Rotarix contained high levelsof PCV1 particles, , these particles were not infectious This confirms the initial assessment that Rotavirus vaccine is safe. These studies were published in the journal Vaccine on the 17th of Janurary 2011.
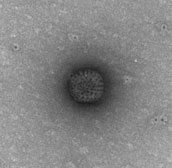 Electron micrograph of a rotavirus particle from the Rotarix vaccine
Source: N. Bannert, RKI
Electron micrograph of a rotavirus particle from the Rotarix vaccine
Source: N. Bannert, RKI
In spring 2010, the Paul-Ehrlich-Institut (PEI) was notified that the vaccine Rotarix, manufactured by GlaxoSmithKline Biologicals, and used for the prevention of gastroenteritis, contained DNA (genetic material) from porcine circovirus type 1 (PCV1) (Victoria JG et al., see below). At that time, millions of children had already been treated with the contaminated vaccine, and no adverse effects had been observed which could have been due to this contamination. "In spite of this, it was important for us to verify whether a potential risk could exist for these vaccinees, which could be caused by the contamination
", explains Prof Klaus Cichutek, President of the PEI. Therefore, scientists atthe PEI immediately started their own experimental studies. With the aid of a specific detection method for the porcine virus, scientists evaluatedthe PCV DNA content in the Rotarix vaccine. In addition, in collaboration with a team of researchers headed by Dr Annette Mankertz, an associate professor at the the Robert-Koch-Institut, they performed assays to detect whether the DNA was packaged into viral particles, and whether these viral particles were infectious. In doing so, they found that although the vaccine contained levels of of PCV1 particles, the latter were not infectious, and therefore did not represent a health risk.
Once the contamination came to light, the manufacturer examined samples from the clinical trial subjects to investigate whther there was evidence for infection of the subjects by PCV1. Infants, even in the clinical trials had been vaccinated with contaminated material. The manufacturer’s tests, also failed to reveal any evidence of PCV1 transmission in the vaccinees. "Regulatory research, with a focus on the safety of medicinal products, constitutes an important pillar of our work as a marketing authorisation agency. It is ourown research activities, in particular which enable us to assess the efficacy and safety of medicinal products reliably and with outstanding expertise
", emphasises Prof Cichutek. "Our own investigations were very helpful in scrutinising and verifying the data submitted by the vaccine manufacturer
", adds Dr Johannes Blümel, Head of the Viral Safety Section at the PEI.
The pig virus was probably introduced into the vaccine by contaminated trypsin used during the manufacturing process. Trypsin, a mixture of digestive enzymes from porcine pancreas is a common reagent used for cell culture and the production of rotavirus vaccine. Even though it is unlikely that the PCV1 particles have a harmful effect, the virus experts of the Paul-Ehrlich-Institut support the development of regulations controllingporcine trypsin used in the manufacture of medicinal products which have been discussed at the European Medicines Agency (EMA). In a press release from the EMA issued on the 18th of November 2010, it was announced that guidelines are to be drafted. PCV-1 does not cause any signs of illness in pigs and is common in pig herds worldwide. Despite extensive experimental studies, PCV infection of humans has never been demonstrated.
Why does Rotarix display a positive risk/benefit balance?
- PCV1 is non-pathogenic, i.e. does not cause disease in humans and animals, and infections in humans are unknown.
- According to the findings of the above mentioned experiments, PCV1 particles found in Rotarix are not infectious.
- Around 100,000 children were vaccinated with Rotarix during clinical studies. More than 68 million doses have so far been administrated worldwide. There is no indication of adverse effects of Rotarix associated with PCV1.
- PCV1 DNA in Rotarix was detected using a new metho combining nucleic acid amplification withstate of the art high throughput DNA sequenceanalysis. This method is suitable for detecting impurities which cannot be identifiedby conventional methods, and would not have been found a few years ago due to methodological limitations.
Publications
Baylis SA, Finsterbusch T, Bannert N, Blümel J, Mankertz A (2011): Analysis of porcine circovirus type 1 detected in Rotarix vaccine.
Vaccine 29: 690-697.
Online-Abstract
Victoria JG et al.: Viral nucleic acids in live-attenuated vaccines: detection of minority variants and an adventitious virus. J Virol. 2010;84(12):6033-6040
Abstract
Press Contact:
Paul-Ehrlich-Institut
Pressestelle
Dr. Susanne Stöcker, Dörte Ruhaltinger
Paul-Ehrlich-Straße 51-59
63225 Langen
GERMANY
Telefon: +49 6103 77 1030
Telefax: +49 6103 77 1262
E-Mail: Presse@pei.de



