Progress in specific immunotherapy of food allergies
Research and regulation of biomedicines at the Paul-Ehrlich-Institut – an unbeatable pair
Allergen specific immunotherapy of food allergies – until now this has been a distant aim. Together with colleagues from Salzburg, Vienna, and Tarragone, researchers from the Paul-Ehrlich-Institut in Langen, Germany, have now developed a vaccine candidate against peach allergies about which they report in the Journal of Allergy and Clinical Immunology. The teams of Dr. Masako Toda and Professor Stefan Vieths have succeeded in generating a hypoallergenic variant of the major peach allergen. This variant possesses almost no allergenicity and is characterised by a strong cellular immunogenicity – a prerequisite for its use as vaccination antigen.
There are still no established therapies against IgE-mediated food allergies – "avoiding the cause" remains the treatment of choice. Desensitisation treatment with protein extracts as in the case of pollen allergies is not established in the case of food allergies since the risk of adverse reactions – which can be as severe as anaphylactic shock – is too high. The development of hypoallergenic variants represents a strategy of minimising the risk of adverse effects in therapy while administrating allergen doses which are as high as possible to achieve induction of clinical tolerance. An approach to optimizing allergen-specific immunotherapy (SIT) in birch pollen allergy is currently under clinical development. SIT aims at inducing peripheral tolerance which is mediated by T-lymphocytes and probably also by "blocking" IgG antibodies. In developing hypoallergenic therapeutics, it is crucial to preserve T-cell reactivity and thus cellular immunogenicity in order to facilitate immune-modulation and thus to make a therapy at all possible.
An international team coordinated by Dr. Masako Toda involving the Division of Allergology, directed by Professor Stefan Vieths has developed an immunotherapy approach on the basis of this strategy. As target antigen they selected Pru p 3, a major allergen in peach allergies frequently causing severe reactions and belonging to the family of non-specific lipid transfer proteins (nsLTPs). nsLTPs are cross-reactive allergens ubiquitously occurring in various fruits, especially in rosaceae plants such as peaches, cherries, apples, as well as in tree nuts, lettuce, grapes and other foods. Although nsLTPs frequently cause serious allergic reactions due their high stability in the digestive tract, their use as a potential allergy vaccine has not yet been studied. Conventional therapy attempts, such as treatments using sublingual immunotherapy with peach extracts, had shown only minor effects in a clinical study. Thus there is an urgent need to develop new treatment strategies.
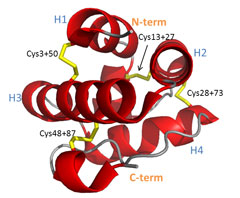 Correctly folded form of Pru P3, the major peach allergen. Intramelecular Disulfide bonds, chemically modified for unfolding, are marked yellow.
Source: Dr. Fötisch, PEI
Correctly folded form of Pru P3, the major peach allergen. Intramelecular Disulfide bonds, chemically modified for unfolding, are marked yellow.
Source: Dr. Fötisch, PEI
"We have developed a folding variant of Pru p 3 and examined its biochemical and immunogenic properties"
, explained Professor Stefan Vieths, who is also the vice president of the Paul-Ehrlich-Institut. "After successfully unfolding the protein structure of Pru p 3, we succeeded in very strongly reducing IgE reactivity and allergenicity, while T-cell immunogenicity is preserved"
, said Professor Vieths. Investigations of IgE reactivity, allergenicity, and T-cell immunogenicity were performed on patients allergic to peach who showed Pru p 3-specific IgE-antibodies. In a mouse model, T-cell immunogenicity of the folding variant could be confirmed. In addition, the authors were able to demonstrate that unfolding the nsLTP had a very strong influence on the stability and antigenic properties of the potential allergen vaccine. The protein structure thus significantly determines the properties of a candidate vaccine antigen. These results are very important for the evaluation of the safety and the mode of action of new allergy immunotherapeutics.
As regulators, PEI staff members evaluate allergen products for the diagnosis and immunotherapy of allergies. First preclinical and clinical approaches exist for the immunotherapy of birch pollen allergies using vaccine candidates with reduced allergenicity. However, no marketing authorisation for such a product has been granted so far and fundamental research work in the field of food allergy is still required. "The scientists of the Paul-Ehrlich-Institut combine expertise from the regulatory field with research in a form which is unique in Europe"
said Professor Klaus Cichutek, President of the Paul-Ehrlich-Institut. "Therefore, we are in a position to contribute to the development of highly efficacious and safe medicinal products with our own basic research"
, Professor Cichutek explained.
The hypoallergeneic Pru p 3 variant developed in the project could be a candidate for the development of a specific immunotherapy against peach allergy with convincing T-cell immunogenicity and a markedly minimised risk of severe adverse reactions.
Further information:
Toda M, Reese G, Gadermaier G, Schulten V, Lauer I, Egger M, Briza P, Randow S, Wolfheimer S, Kigongo V, Del Mar San Miguel Moncin M, Fötisch K, Bohle B, Vieths S, Scheurer S (2011): Protein unfolding strongly modulates the allergenicity and immunogenicity of Pru p 3, the major peach allergen.
J Allergy Clin Immunol 128: 1022-1030.e7.
Online-Abstract
Press Contact:
Paul-Ehrlich-Institut
Pressestelle
Dr. Susanne Stöcker, Dr. Corinna Volz-Zang, Brigitte Morgenroth
Paul-Ehrlich-Straße 51-59
63225 Langen
GERMANY
Telefon: +49 6103 77 1030
Telefax: +49 6103 77 1262
E-Mail: Presse@pei.de



